At-Home STEM Activities: Make your own pH indicator
Yesterday we shared a video demonstration of one of our educators pH-testing the water in our hydroponic garden. The pH scale isn’t just something we find inside a laboratory. We experience the effect of pH every day, all around us—in the things we touch, eat, and even inside our own bodies!
When we measure pH, we are looking at chemistry. At a molecular level, acids have a high concentration of hydrogen (H+) ions, while bases have a high concentration of hydroxy (OH-) ions.
We can determine the pH of an unknown liquid by using an indicator. An indicator is something—usually a liquid or a specially treated paper—that changes color in predictable ways when it touches an acid or a base.
In yesterday’s video demonstration, Educator Faithe mixed a liquid indicator with a sample of plant water and used the color changes to determine that the water had a pH of about 5.5. Another common pH indicator is litmus paper, a specially treated strip of paper that changes color when exposed to acids or bases.
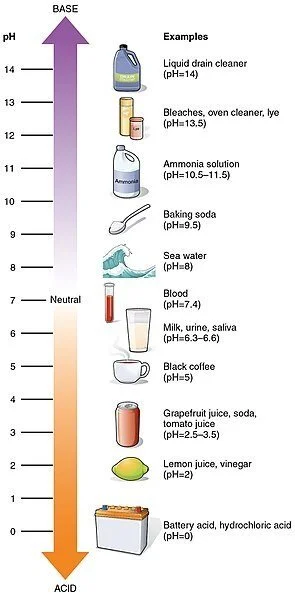
Scientists use something called the pH scale to measure how acidic or basic a liquid is. The liquid is assigned a number from 0 to 14. That number shows where the substance is located along the pH scale.
Any liquid with a pH greater than 7 is a base. The more basic—or alkaline—a liquid is, the higher its number. Our blood is slightly basic. Some household cleaners have quite a high pH.
Substances with a pH of 7 are neutral. This means they are neither an acid nor a base.
Any substance with a pH less than 7 is called an acid. The stronger—or more acidic—a liquid is, the lower its number. You’ve probably experienced the effects of an acid if you ever tasted a lemon and puckered! That sour taste is because lemon juice has quite a low pH.
With this easy at-home activity, we’ll create our own pH indicators, using nothing little more than white paper and a red cabbage! Try creating your own cabbage-water pH indicator, either as a liquid or a paper strip.
Important Note: Acids and bases can be dangerous! For your safety:
Adult supervision/assistance is required for this activity
Be sure to wear safety gloves and goggles, and exercise extreme caution when working with chemicals or on the stove. NEVER heat or mix unknown chemicals!
Wash your hands and all equipment/dishes used thoroughly after this project
Materials:
Red cabbage
Pan & stovetop
Colander/strainer
White paper or cardstock
Scissors
Selected edible or household liquids—we used: drain cleaner, diluted dish soap, milk, cranberry juice, baking soda in water, black coffee, lemon juice, and laundry detergent
Process:
Part One
Slice the cabbage into chunks or slivers. Place about 1/2 the cabbage into a pot of water, and bring to a boil. Boil for 15 to 30 minutes. (The longer you boil the cabbage, the more it will leech into the water.)
After boiling, remove the pot from heat and allow it to cool. Then use a strainer to carefully separate the solid cabbage fragments from the liquid. You may place the solids aside (dinner, anyone?): for the sake of this project, we are interested in the remaining blue-to-purple liquid. This cabbage water will be our pH indicator! The pH of cabbage is naturally very neutral, with a pH around 7.
Part Two
Cut your white paper into several strips. (We tried regular paper and some leftover note cards. The thicker paper results in a sturdier pH indicator strip, but takes longer to dry.)
Dip or submerge the paper strips into your cabbage water, then pull out and allow to dry. This will be a messy process! (We placed our wet paper strips onto paper towels, and helped the drying process along with a hair dryer on a low setting.)
Part 3
Pour small amounts of household liquids into individual containers, and label if needed to keep track of them. These are your test substances.
Part Four—The Experiment
Before trying your pH indicator strips, ask yourself whether you expect each of the test substances to be acidic or basic, based on what you already know about what they are or how they are used. Scientists call this process forming a hypothesis.
Dip the end of your indicator strip into each test substance, making sure not to spill. What happens to the paper?
Remember that pH indicators react predictably when they interact with acids or bases. Cabbage water turns red for strong acids (substances with very low pH), purple or violet for weaker acids, purple to blue for neutral substances (with a pH around 7), blue to green for bases (substances with pH over 7), and yellow for strong bases (substances with very high pH).
Alternate Experiment: use your cabbage water as a liquid indicator
Fill a glass about 3/4 full of plain water. Add several drops of cabbage water and stir to mix well. You want your cabbage water to be extremely diluted—so that your indicator liquid has only a very light blue tint.
Add about 1/4 cup of a test liquid to the glass of indicator liquid and stir. What happens to the liquid inside your glass?