Distance Learning Module: Nutrient Cycling
In honor of National Wildflower Week, this week’s Distance Learning offerings are focusing on the plant world.
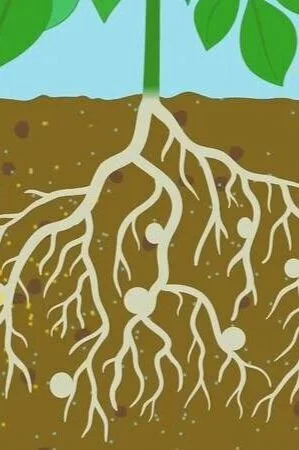
Plants use their roots to absorb nutrients from the soil in which they are growing. The process of nutrient cycling captures elements such as nitrogen from the atmosphere, converts them into a usable form for plant nutrition, and returns those elements back to the atmosphere when the plant dies and decomposes. Learn more about the “how” and “why” of nutrient cycling here!
plant growing in sandy ground at kennedy space center in florida (image credit: NASA/Bill White)
Plant Nutrition
Plants require certain minerals from the environment in order to perform the functions of life, such as making food through photosynthesis and building the proteins needed to grow. Unlike us, plants can’t just open the fridge and pull out a snack when they’re hungry! Plants use their roots to absorb nutrients from their growing medium (which is usually soil—but the growing medium can also be water, like in the hydroponic garden in our Lunar Colony exhibit). Nutrients that plants obtain from their growing medium include:
plants use their roots to absorb nutrients from the soil (image courtesy brittanica.com)
macronutrients—chemical elements that plants need in large quantities to perform basic life functions, including: nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), sulfur (S), magnesium (Mg), carbon (C), oxygen (O), and hydrogen (H)
micronutrients—which are required only in trace quantities, and include: iron (Fe), boron (B), chlorine (Cl), manganese (Mn), zinc (Zn), copper (Cu), molybdenum (Mo), and nickel (Ni)
Nutrient Cycling
There is only a finite (limited) amount of minerals available in the natural environment. Nature has to recycle these nutrients, so that they can be used and re-used by all of the organisms that make up an ecosystem.
The nutrient cycle is a system where energy and matter are transferred between living organisms and non-living parts of the environment. Animals and plants consume nutrients in order to live, and the nutrients are released back into the environment via death and decomposition. They are then available once again for other living things to consume and use for their own life functions.
diagram courtesy of usgs
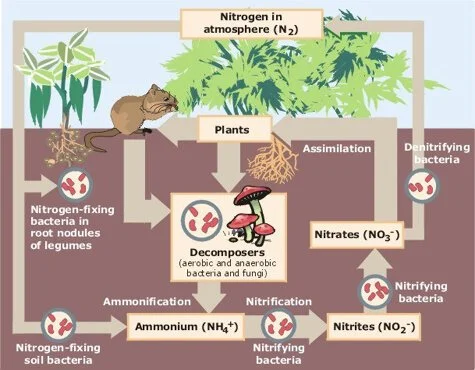
The Nitrogen Cycle
To better understand how nutrient cycling works, let’s look at the nitrogen cycle. Nitrogen is one of the primary nutrients critical for the survival of all living organisms. Without it, plant growth would be extremely limited. Nitrogen is the most abundant element in our atmosphere, in the form of dinitrogen gas (N2)—but it’s not useful for plants in this form. For plants to access nitrogen in the environment, it must first be converted to ammonia (NH3). The nitrogen cycle includes several steps:
Fixation: Special bacteria (microorganisms) in the soil change nitrogen from the air into ammonium (this process is called “fixing” nitrogen)—the first step in making the nitrogen usable for plants
Nitrification: Microorganisms further convert ammonium into nitrates—this is the form that plants actually take in as nutrients
Assimilation: Plants absorb nitrates from the soil into their roots. The nitrogen is then used to make amino acids, nucleic acids, and chlorophyll. An animal might then go on to eat the plant, re-assimilating the nitrogen for its own nutritional needs
Ammonification: This is part of the decaying process. When the plant (or the animal that ate it) eventually dies, decomposers like fungi and bacteria in the soil turn the nitrogen back into ammonium
Denitrification: The final step, in which other bacteria convert the ammonium compounds back into nitrogen gas (N2), which is then released back into the atmosphere to begin the cycle again
Perhaps the loveliest aspect of the nitrogen cycle is that it is necessary for life on Earth, and yet it depends on all the organisms within an ecosystem—from the “lowliest” microscopic organisms in the soil to the largest animal predators!
graphic courtesy of u.S. environmental protection agency
Why It Matters
Proper nitrogen levels are a vital aspect of maintaining the delicate balance of a living ecosystem. Without enough nitrogen we couldn’t survive—but too much, and it’s toxic!
When plants lack nitrogen, they become yellowed, grow poorly, and produce smaller fruits and flowers. Some farmers help their plants grow by adding chemical fertilizers containing nitrogen, phosphorus, and other elements to their fields. This helps humans to produce food in the amounts that we need to survive—but excessive nitrogen can actually be harmful to life.
eutrophication in action: toxic algae bloom in the potomac river (image credit: wikimedia commons/ Alexandr Trubetskoy)
When there is more nitrogen and phosphorus in the soil than is needed for use by plants, the unused minerals build up. Eventually, the excess minerals enter waterways during rain showers, snow melting, and gradual leaching into the groundwater. High levels of nitrogen and phosphorus can cause gross, smelly, uncontrolled algae blooms to grow in bodies of water (this is called eutrophication), causing “dead zones” where nothing can survive.
Over-fertilizing can kill or stunt the growth of plants, disrupt the pH and salinity of the soil, and create long-term barriers to healthy plant growth. When nitrogen fertilizer is applied to the soil surface, nitrous oxide can be released into the atmosphere, where it acts as a potent greenhouse gas, trapping heat and contributing to climate change.
corn fields in a drought (image courtesy of union of concerned scientists)
What Can Be Done?
Fortunately, farmers and gardeners can take steps to avoid nutrient pollution!
vegetable garden at the remick country doctor museum & farm, Tamworth, NH (image courtesy faithe miller lakowicz)
Eliminating or reducing the use of fertilizers keeps too many minerals from entering the soil for your plants to use
Planting cover crops such as legumes and clovers can reduce soil erosion and help to capture and fix nitrogen from the air (reducing the need for chemical fertilizers)
Controlling irrigation prevents excess nutrients from leaching and running off into water sources
Native plants are better adapted to local conditions, reducing the need for additional fertilizer and maintaining local biodiversity. Consider planting a native garden!
See this site from the U.S. Environmental Protection Agency for many tips about how you can reduce nutrient pollution in your own home and yard:
Play a Game
Round off today’s lesson with these nutrient cycling games, courtesy of the Nutrients for Life Foundation (nutrientsforlife.org):
image courtesy of wikimedia commons/Rochelle Vergara
You provide a corn crop with the nutrients it needs to grow: water, nitrogen, phosphorus and potassium. Try to keep all four dials in the green!
Test your knowledge as you help Tom or Jamie work their way through the nitrogen cycle. Explore two different scenarios, answering questions about the nitrogen cycle in corn fields or a tomato garden.